Heart Comprehension Sheet
1. Where is your heart located?
To the left of the center of your chest.
2. What does your heart do for your body?
It is a pump that moves blood through your body.
3. How is the left side of your heart different from the right side?
The right side receives blood from your body and pumps it into your lungs.
The left side receives blood from the lungs and pumps it into your body.
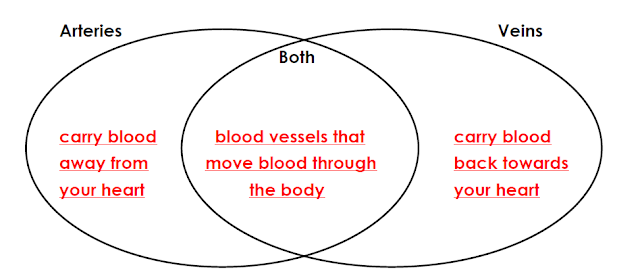
4. Complete the Venn diagram to compare and contrast the functions of
arteries and veins.
5. What are some things you can do to keep your heart healthy?
Eat healthy foods, such as whole grains, fruits, vegetables, and lean proteins.
Exercise to keep your blood pumping hard.
Bill Nye: “Heart”
Video Listening Guide
1.
Your heart is a muscle
that is about as big as your fist.
2.
Your heart has 2_ sides
and it has 4__ chambers.
3.
Valves are structures that keep
the blood flowing in one direction.
4.
The normal heart beats between 60
- 100 beats per minute.
5.
Exercise makes your heart beat faster because the muscles need more oxygen.
6.
In the right side of your heart wall is a patch of nerves called
the sinoatrial
that sends
electrical signals to your heart.
7.
The muscles in your arms and legs have veins that look like stripes.
8.
Heart muscle looks like a combination of striated and smooth muscle.
9.
You can make your heart muscle stronger by exercising.
10. When you cut a blood vessel,
your body forms a blood clot
to prevent blood from leaking out.
11. How is the blood pumped in
the body during heart surgery? Heart and lung machine.
12. When you stand up quickly
you sometimes feel “light-headed” because:
of gravity. Blood stays lower in your body and the heart
cannot pump it up fast enough.
13. The kind of fat that is bad for you is animal fat because it can clog
your arteries.
Study Guide The
Circulatory System
Quiz
on Tuesday, June 7th
1. Know
the parts of the circulatory system, their structures and functions. (Your
Chart)
2. Study
page 29 of your text. Know what the
blood cells are and their functions.
3. Review
the Bill Nye “Heart” Video Listening Guide.
4. Know
“Your Heart” comprehension sheet and questions.
Bill Nye Blood and Circulation Video
Click here if video doesn't play:
Bill Nye - Blood and Circulation by curtdogg85
Click here if video doesn't play:
Bill Nye Heart Video
Bill Nye The Science Guy 4x16 - Heart by BillNyeTheScienceGuyTV
Body Works Lesson 8
Click here if video doesn't play:
Bill Nye - Blood and Circulation by curtdogg85
Click here if video doesn't play:
Bill Nye Heart Video
Bill Nye The Science Guy 4x16 - Heart by BillNyeTheScienceGuyTV
The Structures
and Functions of the Major Organs of the Circulatory System
Major Organ
|
Structure
|
Function
|
Heart
|
About the
size of your closed fist. It is
divided into two parts by a sheet of muscle called a septum.
|
Heart
valves work like one way doors to keep the blood in the two parts from
mixing. Your heart is a powerful
machine that can pump blood to and from the farthest regions of your
body. It has to push your blood
through 80 000km of capillaries alone, not counting the larger arteries and
veins.
|
Blood
Vessels
|
Three kinds
are: arteries, capillaries, veins
|
Carries nutrients
and oxygen to your cells and takes wastes, like carbon dioxide out of your
cells.
|
Arteries
|
Thick
muscular vessels. The inner layer of
an artery is “leak-proof”. The outer
layer is stretchy. In between is a
layer of muscle.
|
Arteries
carry blood away from the heart to the rest of the body.
|
Veins
|
Most veins
have valves that act like one-way doors to keep the blood moving along toward
the heart. Veins have the same layers
as arteries but they have thinner walls, since the blood pressure in veins is
much lower. Veins join together in
larger and larger veins until they reach the heart.
|
Most veins
carry oxygen poor blood back to the heart from every part of the body.
|
Capillaries
|
The
smallest of the blood vessels.
|
Capillaries
take oxygen and nutrients from the arteries to the cells. Then they take the wastes from the cells
back to the veins.
|
Blood
|
The red
fluid that circulates in the blood vessels.
Made up of red blood cells, white blood cells and platelets.
|
A road
system built to transport the good your body needs from place to place. Also see page 29 in text.
|
Body Works Lesson 8
What happens when you breathe?
1.
What is your diaphragm? How can you locate it on your body?
Your
diaphragm is a strong sheet of muscle which separates your lungs from the
organs below it. I can feel it move when
I breathe in and out.
2.
Some people with hay fever, asthma or
allergies to things like animal fluff and dust mites can have difficulty with
their bronchial tubes which causes problems with their breathing. What is happening to their bronchial tubes
and what do they do about it?
The
bronchial tubes close up and they have difficulty breathing. To help them breathe more easily, some people
have to use an inhaler regularly.
3.
What should you do when you feel the
urge to sneeze? Why?
Sneeze. Sneezing helps get rid of harmful substances.
4.
What is carbon monoxide and how is it
produced?
Carbon
monoxide is a deadly gas produced by any motor that runs on gasoline.
5. The dangers of smoking are printed on every cigarette package, yet many, many people smoke. Make a list of reasons why you think people ever start to smoke at all.
Answers may vary. Peer pressure, to be "cool", curiosity.
Respiratory System Study Guide
Quiz
Date: Monday, May 9th
Ø Know the
structures and functions of the respiratory system. (Your chart)
Ø Read
pages 22-25 in textbook.
Ø Be able
to label the respiratory system with these parts:
nasal passage
trachea
lung
diaphragm
bronchial tubes
Ø Read and
understand the questions for Lesson 6, Activity 1 and What Happens When You
Breathe sheet.
Lesson 6; Activity 1 Sheet Answers
1.
2. Blood
has to go to the lungs to allow for gas exchange. Blood picks up oxygen from the lungs in
exchange for carbon dioxide.
3. No,
because the lungs do not have muscles themselves.
4. Carbon
dioxide goes into a balloon when you blow it up. When we exhale, we are getting rid of
carbon dioxide.
Answers: 1. F, 2. T, 3. F, 4. F, 5. F, 6. T, 7. D, 8. B, 9. D, 10. A
Bill Nye Respiration
Canadian Scientist Assignment: Due May 2nd.
Study Guide The Digestive System
1. Know
the parts of the digestive system, their structures and functions.
2. Know
what digestion is and be able to label the digestive system. (Lesson 4;
Activity 1 sheet)
3. Review
the Bill Nye fill in sheet on digestion.
4. Review
pages 14–21 in your student text.
MY QUIZ IS ON: __Monday, April 11th,
2016
The Digestive Process
·
Digestion
is the mechanical and chemical process that breaks food down into substances
tiny enough to be absorbed by the body’s cells.
·
The process begins in the
mouth with chewing. The teeth bite and
tear the food and crush and grind it.
Saliva, which flows from glands in the mouth and cheeks, contains
ptyalin, an enzyme that breaks starches into sugars.
·
When you swallow, food
enters the digestive tract – an eight metre tube that runs from mouth to anus.
·
In the esophagus, food
travels down the chute like passage from your mouth to your stomach with the
help of a wavelike action called peristalsis.
·
In the stomach, swallowed
food is churned and mixed with gastric juices which prepare the food for
further breakdown. The partially digested
food, called chyme leaves the stomach and enters the small intestine.
·
About 95% of the digested
nutrients are absorbed into the bloodstream from the small intestine. All that remains are waste materials and
mineral-rich water.
·
The elimination of wastes
through the large intestine completes the digestive process. Most of the water is reabsorbed by the
intestinal lining, but the solid substances that cannot be used continue down
the large intestine to be eliminated from the body.
·
At the end of the digestive
process, the feces (poop) pass into the rectum, the short tube at the end of
the large intestine.
·
Strong muscles hold the anus
closed until enough water builds up, and until a conscious decision is made to
defecate (poop).
Lesson 4; Activity 1 Answers
Food provides the body with energy it
requires. The nutrients the body needs comes from the food and drink we
consume. This process of change is known as digestion. When food enters
the mouth, fluids start changing the
starches into sugar that the body can digest with the help of the saliva. The food continues
down the esophagus. Muscular movements
squeeze and push it down to the
stomach.
Once in the stomach, some food remains here for awhile. Other food
particles are further broken down by gastric juices. These juices are
made up of water and chemicals that soften the food and kill the
bacteria. This process takes approximately 3 to 4 hours to occur.
Bill Nye Nutrition
Bill Nye The Science Guy Season 4 Episode 2... by BillNyeTheScienceGuyTV
Bill Nye Digestion
Bill Nye The Science Guy Season 1 Episode 7... by BillNyeTheScienceGuyTV
Bill Nye:
Digestion KEY
1. You
aren’t what you eat; you’re what you DIGEST!
2. Our
bodies are like an ENGINE that
runs on fuel.
3. FOOD is fuel for your body.
4. Food that
we eat goes down our ‘food tube’, or ESOPHAGUS, into our STOMACH.
5. When you
get hungry, your stomach muscles CONTRACT to remind you to give it food to digest.
6. Your
stomach contains a strong acid, HYDROCHLORIC acid, to
help you break down food.
7. How long
does it take to break down food in the acid in our stomachs? SEVERAL HOURS
8. Why
doesn’t the acid in our stomachs hurt our stomach? A MUCUS LINING IN THE STOMACH PROTECTS FROM THE ACID
9. We eat
about 1 KG of food
and drink about 3 L of water
every day!
10. Where do the chemicals from our food get absorbed into
the body? IN THE
SMALL INTESTINE
11. For an
adult, the small intestine is about 7 M long and the large intestine is about 2M long!
12. Omit
13. The muscular contractions
that move food down your throat to your stomach (even if you’re upside down or
in space!) is called PERISTALSIS.
14. You make about 1 L of saliva a day!
15. Your TEETH grind up your food so your body can access the
nutrients in it.
Bill Nye Buoyancy
Quiz on Tuesday, March 1st, 2016
What’s the Matter?
Study Guide Name:
Ø Review
definitions (Green words from pages 26-37)
Ø Review
all notes and worksheets for lessons 8-11
Ø Review
Lessons 8-11 in textbook
Concentrate on:
·
The difference between a physical
and chemical changes and be able to give examples of each.
·
Volume and mass during chemical
and physical changes and whether matter is created or destroyed.
·
The physical states of water and
know how to draw them.
·
When reversible and
non-reversible changes occur in physical and chemical changes.
Your test will have:
v Matching
definitions
v One
complete the chart activity
v Short
answer questions
v Fill
in the blank
1. Physical Change: A change in which the particles of a substance
are rearranged but do not change.
2. Evaporation: A change from a liquid to a gas.
3. Reversible Change: Change that occurs in matter that can be
reversed. Evaporation and condensation
are examples of reversible changes.
4. Non-reversible Change: A change that occurs in matter that cannot be
reversed. Burning wood and frying an egg
are examples of non-reversible changes.
5. Chemical Change: Change in matter that produces a new
substance. Baking a cake mixture is an
example of a chemical change.
6. Distillation: A method of separating a pure liquid from a
mixture.
Lesson
8: Is Matter Created or Destroyed in a
Physical Change?
Ø A
physical change is when the particles of a substance are rearranged, but do not
change. During a physical change, you do
not lose matter and no new material is created.
Ø The
volume of a substance may change, but the particles of that substance do not
change. For example, when you dissolve
salt in the water, its smallest particles remain the same. But these particles are now separated from
each other and surrounded by water particles.
Only the volume changed not the number or type of particles that make up
salt or water.
Ø Another
example is breaking a vase. After the
vase broke, it was in many pieces and took up less space, but the particles of
the vase remained the same.
Ø Mass
is the number of particles that make up a substance and this does not change
during a physical change.
Lesson 9:
Do Changes of State Affect Water’s Mass or Volume?
Ø When
water freezes, its molecules move to a more open spread apart position.
Ø Therefore,
frozen water takes up more space than liquid water.
Ø This
is what causes ice cubes to float. They
are less dense than liquid water.
Ø Density
is the ration of an object’s mass to its volume.
Ø Changes
of state help us in our everyday lives.
For example, refrigerators, steam trains, ice rinks.
Lesson
10: What Are Reversible Changes?
Ø Water
can change state from a solid to a liquid to a gas and back to a liquid.
Ø Melting
is a change from a solid to a liquid and freezing is the reverse of this
change.
Ø Evaporation
is a change from liquid to gas and condensation is the reverse of this change.
Ø These
changes are called reversible changes.
Ø Reversible
changes are usually, but not always, physical changes. For example boiling water, which is a
physical change, is reversible. Sanding
wood is also a physical change, but it is not reversible.
Ø When
a change occurs in matter that is not reversible, it is called a non-reversible
change. For example burning wood or
rusting metal.
Ø A
chemical change is a change in matter that produces a new substance. Sometimes they are non-reversible, but not
always.
Lesson
11: What Happens During a Chemical
Change?
Ø In
chemical changes at least one kind of matter is produced that was not there
before the change.
Ø The
key things to look for in a chemical change are the signs of one or more new
substances after the change.
Ø The
new substance may have a different colour, texture, smell, or taste. There could also be new characteristics such
as different boiling and melting points, or giving off heat or light.
Bill Nye - Chemical Reactions by curtdogg85
Quiz Monday, January 25th, 2016
What’s the Matter?
Study Guide Lessons 1-6 Name:
Ø Review
definitions for lessons 1-6
Ø Review
all notes for lessons 1-6
Ø Review
Lessons 1-6 in text
Ø Review
Matter sheets.
Concentrate on:
·
The 3 states of matter and how
their particles look. (p.8)
·
What is a fair test? What is a variable? What is an insulator?(p.10)
·
Know the properties of
matter.(p.12)
·
How does matter behave when
heating or cooling?(p.16)
Your test will have:
v Six
matching definitions
v Ten
fill in the blanks (with word bank)
v One
complete the chart activity
v Short
answer questions
What’s The Matter Notes
Lesson 1: The
Fact of the Matter: Things Change
Ø Steps
of Scientific inquiry:
1.
Ask questions about the problem
to help you define it.
2.
Make a hypothesis – a statement
about a possible answer of solution to the problem.
3.
Design an investigation to test
your hypothesis.
4.
Collect materials you will need.
5.
Conduct your investigation.
6.
Record the results of your
investigation.
7.
Draw conclusions from your
results.
8.
Communicate your results and
conclusions to others.
9.
If possible, relate what you have
learned to the world outside the classroom.
Lesson 2:
What is Matter Made Of?
Ø Everything
in the universe is made of matter. Matter
is made up of tiny particles called atoms.
Ø Matter
can be anything that takes up space and has mass.
Ø Matter
changes every day, everywhere.
Ø Gases
and liquids behave as they do because of how their particles move when heated.
Ø Matter
exists in one of three states: solid, liquid, or gas.
Ø Which state it is in
depends on the arrangement of the tiny particles that make it up.
Ø Solids
have a definite volume and hold their shape.
The molecules are very close together.
Ø Liquids
have a definite volume and take the shape of their container. Their molecules move more freely.
Ø Gases
have no definite volume and take the volume and shape of their container. Their molecules are very far apart.
Ø Increasing
the temperature breaks up the pattern of particles and they start to move
faster and faster. Heat makes particles
expand. Cooling particles makes them
contract.
Lesson 3: What
Is a Fair Test?
Ø When
scientists conduct tests, or experiments, they must be sure that their results
are as accurate and meaningful as possible.
Ø In
a fair test, all the variables, or factors that can affect the results of an
experiment, are controlled except the one under investigation.
Lesson 4: What Are Some Properties of Matter?
Ø Scientists
use properties to tell the difference between different kinds of matter.
Ø Some
common properties we use to describe matter include texture, hardness,
strength, flexibility, buoyancy and solubility.
Ø Just
as a butterfly has a life cycle, a product can also have a life cycle, for
example paper. (Page 14-15)
Lesson 5: How Does Matter Behave?
Ø All
matter has the ability to change states.
Ø As
matter heats, the particles begin to move faster, changing from a solid, to a
liquid to a gas.
Ø As
matter cools, the particles begin to slow down, changing from a gas, to a
liquid to a solid.
Ø When
changing between states, there is no effect on its mass.
Lesson 6: How
Can Matter Be Mixed Together?
Ø A
solution is a mixture that forms when one substance dissolves into another.
Ø One
of the factors that affect the rate at which something dissolves is
temperature.
Ø A
solute is the substance that dissolves.
Ø A
solvent is the substance the solute dissolves in.
Ø One
of the factors that affect the rate at which something dissolves is
temperature.
Ø An
increase in temperature causes particles to move faster and move apart. When the solute is a solid, the increased
spaces between water molecules make more room for particles of solute, so the
solute dissolves faster.
Ø A
solvent has a limit of how much solute it can absorb. Once that point is reached, the solution is
said to be saturated and the solute will no longer seem to disappear.
Ø Water
is the most commonly used solvent to test for solubility because it is a clear
substance and you can see the solubility better.
Matter Definitions
1. Matter: Everything in the universe is made up of
matter. Matter is anything that takes up
space and that has mass.
2. Mass: The number of particles that make up a
substance.
3. Particles: Small parts (atoms and molecules) that make
up matter.
4. Volume: The amount of space that matter takes up.
5. States: The tree forms of matter: solid, liquid or gas.
6. Friction: The rubbing of one surface against another.
7. Fair Tests: Exploration carried out under strictly
controlled conditions so results are reliable.
8. Variables: Factors that can affect the results of an
experiment.
9. Insulator: Material that prevents the flow of heat,
sound, and electricity.
10. Properties: Qualities or characteristics of a material,
such as mass or colour.
11. Texture: The way a surface feels to the touch, that is
rough, smooth, slimy, and so on.
12. Hardness: How hard a substance is.
13. Strength: The power or force of a substance; the state of being strong.
14. Flexibility: Ability of a substance to blend easily
without breaking.
15. Buoyancy: The ability to float or rise to the top of a
liquid or gas.
16. Solubility: How well matter can dissolve in other matter.
17. Solution: A mixture of one substance dissolved in
another substance.
Bill Nye Phases of Matter
https://www.youtube.com/watch?v=45YNZjYfHb4
Bill Nye Wind
https://www.youtube.com/watch?v=X6NIlMNlTlk
Felix Baumgartner's supersonic freefall from 128k' - Mission Highlights
https://youtu.be/FHtvDA0W34I
Weatherwise Study Guide Test: Friday, November 13th
1. Review
all notes given for lessons 1-10 (Also available on my blog)
2. Know
the definitions for:
Ø Weather
Ø Climate
Ø atmosphere
Ø mesosphere
Ø pressure
Ø air
pressure
Ø stratosphere
Ø thermosphere
Ø troposphere
Ø ozone
Ø UV
rays
Ø water
vapour
Ø condense
Ø evaporate
Ø front
Ø barometer
Ø greenhouse
gases
Ø pollutant
Ø wind-chill
factor
3. Review
the types of clouds, especially cirrus, cumulus, and stratus.
4. Know
the water cycle and be able to draw, label and explain it using the words
condensation, evaporation, precipitation and runoff.
5. Explain
why the weight of Earth’s atmosphere doesn’t bother us even though there is a
half a ton of pressure on our heads every day. Understand that there is almost
no air pressure in space and what would happen if a human didn’t wear
protective suits.
6. Know
the layers of the atmosphere.
Weather Notes
What is the Atmosphere?
Ø The
atmosphere blankets the Earth with an invisible mixture of gases. This mixture is known as air.
Ø Air
is all around you.
Ø Air
has no shape, colour, smell or taste.
Ø Air
takes up space and has weight.
Ø Air
is made of very tiny particles. The
particles at the top of the atmosphere are far apart without much weight on
them. Air particles at the bottom of the
atmosphere are pressed close together by the weight of the air above them.
Ø The
air above you puts about half a tonne of weight on the top of your head.
Ø The
air over your head does not smash you flat, because you have air inside you
that pushes back against the air outside you.
Ø Air
pushes in all directions all the time.
How Does the Sun Affect the Atmosphere?
Ø The
atmosphere acts as a shield, keeping some of the sun’s energy from getting to
Earth’s surface.
Ø Earth’s
atmosphere does not block light energy.
Ø The
atmosphere traps solar heat and protects our planet from meteor showers, and
harmful UV and cosmic rays.
Ø The
radiation that is absorbed by Earth’s surface is then radiated upward as heat
energy.
Ø Clouds
keep this heat energy on Earth and acts like a blanket keeping our planet warm.
Ø The
more UV rays reach the Earth, the quicker skin can be damaged. It can also
damage your eyes and cause skin cancer.
Ø When
oxygen in the atmosphere comes in contact with UV rays, it changes into another
form of oxygen called ozone. Ozone
absorbs UV rays.
Ø Air
pollution destroys the ozone layer and because of this, more UV rays are
getting through to Earth than 40 years ago.
How Do
Clouds and Rain Form?
Ø Water
vapour in the atmosphere becomes a cloud when it cools and comes into contact
with tiny particles of dust.
Ø There
are three basic kinds of clouds:
ü Cirrus:
light, feathery and formed of ice crystals.
ü Cumulus: puffy, rounded and are called “fair weather”
clouds.
ü Stratus:
layered, spread out and form on a damp, foggy day.
Ø You
will often see clouds that look like combinations of these clouds: Stratocumulus,
Cirrostratus, Cirrocumulus, Altocumulus, Altostratus, Nimbostratus, Cumulonimbus
Ø The
word “nimbus” means “rain cloud,” and nimbus clouds are dark grey.
Ø The
word “alto” means middle level.
The Water
Cycle: describes
the movement of water.
Ø
In the water cycle, water from oceans,
lakes, swamps, rivers, plants, and even you, can turn into water vapor.
Ø
Water vapor condenses into millions of
tiny droplets that form clouds.
Ø
Clouds lose their water as rain or
snow, which is called precipitation.
Ø
Precipitation is either absorbed into
the ground or runs off into rivers.
Ø
Water that was absorbed into the ground
is taken up by plants.
Ø
Plants lose water from their surfaces
as vapor back into the atmosphere.
Ø
Water that runs off into rivers flows
into ponds, lakes, or oceans where it evaporates back into the atmosphere.
Ø
The cycle continues
What
Causes the Wind?
Ø Temperature
changes help cause wind. Lighter air is
pushed upward by heavier air flowing in underneath it.
Ø Warm
air is lighter than cool air and rises up.
Cool air is heavier and sinks.
Ø An
area where the air is heavy (cool) is a high-pressure area.
Ø An
area where the air is light and rises (warm) is a low-pressure area.
Ø Air
moves from a high-pressure area into a lower-pressure area. If the two areas are close together, the air
will move fast.
Ø Wind
is named for the direction from which it blows.
Example: a west wind blows from west to east.
How Do
Storms Form?
Ø Sometimes
a strong, fast column of rising warm air starts spinning. It forms a tube of air that hangs from the
thunderstorm like an elephant’s trunk.
This is called a tornado.
Ø A
tornado acts like a vacuum cleaner, sucking in air at the bottom and whirling
it upwards, picking up anything in its path.
Ø No
one knows why tornados form in some storms and not in others.
Ø Thunderstorms: Giant cumulonimbus clouds can rise up
sometimes reaching the beginning of the stratosphere. Warm air rising inside the cloud carries
water vapour up from the Earth’s surface.
At the same time, condensed droplets join together and fall as
rain. All the different forces in motion
cause high winds and lightning.
Ø Lightning
and thunder form when, particles with an electric charge zigzag from the cloud
toward Earth. Particles with a different
electric charge move upward from Earth.
The two charges meet and a powerful electric current begins to flow
between the cloud and Earth. Lightning’s
heat causes air to expand. Cooler air
movies in. The air movement starts sound
waves that you hear as thunder.
How Do
Weather Systems Move?
Ø Masses
of air move across the surface of Earth, pushing against each other.
Ø The
axis is an invisible line through Earth from the North Pole to the South Pole.
Ø Warm
air first travels from the equator towards the North and South poles. As air cools down it flows back towards the
equator.
Ø Most
of the time, weather in Canada moves from west to east.
Ø Earth’s
axis tilts and the direction of the tilt does not change. And the surface of the Earth is curved. The sun’s energy rays strike different places
at different angles during the year.
Ø Climate
is determined by the amount of the sun’s energy the place gets, its distance
from oceans, wind patterns, and the shape of the land.
Ø Winds
push giant air masses across the surface of the Earth until they run into other
air masses. This is a front.
Ø You
can expect a change of weather when a front moves through.
Ø Warm
fronts are masses of warm air that move forward against masses of cold air.
Ø Cold
fronts are masses of cold air that move forward against masses of warm air.
How Do
People Predict Weather?
Ø There
was no reliable record of daily weather patterns until the beginning of the
twentieth century.
Ø Technology
plays a big part in weather forecasting today.
ü We
use satellite pictures to see where clouds are and how they are moving.
ü We
use radar to find out where there is precipitation. The radar beam travels through the atmosphere
and if it hits a rain or snow sized droplet, it echoes back.
ü Weather
stations collect data such as air temperature, dew point temperature, air
pressure, wind speed, and wind direction.
ü Weather
balloons are sent into the troposphere and collect all kinds of measurements to
help predict the weather. They are programmed
to radio the information back to a weather station.
Ø In
Canada, all this information is collected and sent to supercomputers at the
Canadian Meteorological Centre in Montreal.
Ø The
supercomputer uses the information to create a model of the atmosphere for the
next five days around the world.
Ø Meteorologists
are people who study weather.
Ø A
barometer shows changes in air pressure.
A fall in air pressure often precedes a storm.
How Does
the Weather Affect You?
Ø Before
there were organized weather forecasts, people paid close attention to the sky,
the clouds, and the feel of the air, the winds, and the temperature to forecast
the weather.
Ø The
type of materials a builder uses to build a home is dependent on climate.
Ø Heat
makes materials expand and cold makes materials contract.
Ø In
a Canadian climate, builders need materials that don’t expand or contract very
much because we can have very hot summers and very cold winters.
Ø Materials
that do expand and contract a lot can break apart which is not something you
want to happen to your home.
How Could
Greenhouse Gases Change the Atmosphere?
Ø The
gases in the stratosphere that are good at trapping heat are the greenhouse
gases.
Ø Greenhouse
gases keep the Earth’s atmosphere warm enough for humans and other living
things to survive.
Ø Carbon
dioxide and water vapour are the most common greenhouse gases.
Ø Acid rain is rain containing acids that
form in the atmosphere when pollution from car exhausts and other industry
emissions combine with water.
Ø The major cause of acid rain is car
exhaust.
Ø Carbon dioxide is not a harmful gas by
itself. It does not hurt you to breathe
carbon dioxide as long as you have enough oxygen to breathe at the same time.
Ø Plants need carbon dioxide to grow.
High above
the Earth is the exosphere, the final layer of our atmosphere. This layer
extends into space. Solar winds compress it and push it down. When the winds
are still, this layer can extend more than 6,000 miles into space. The most common molecules within Earth's exosphere are those
of the lightest atmospheric gasses. Hydrogen is present
throughout the exosphere, with some helium, carbon dioxide,
and atomic oxygen near its base.
Because it can be difficult to define the boundary between the exosphere and
outer space, the exosphere may be considered a part of outer space.
Layers of the Earth's Atmosphere
The Earth's Atmosphere
The layer of air that surrounds Earth is called the atmosphere.
The atmosphere looks like a thin blanket surrounding the planet.
The earth is made of billions and billions of gas particles.
Earth's air contains many gases. Look at the graph.
What percentage of the air is nitrogen? 78
oxygen? 21
argon? 0.93
carbon dioxide? 0.035
Water vapor in the atmosphere ranges from 0-4%.
Earth's atmosphere also contains dust particles. What kinds of things do you think make up the dust particles in the air?
(dirt, soot, pollen grains, meteor remains, etc.)
Dust particles provide a surface for water vapor to condense on so it can become precipitation such as rain, snow or hail.
Another gas that exists in tiny quantities is ozone .It
is made of three oxygen molecules (rather than 2 in oxygen gas.)
Most ozone is in the layer that is 10-50 km (6-30 miles) high. Most
ozone is in the stratosphere layer. Ozone protects life on Earth by absorbing some of the suns's harmful rays.
©
Bill Nye Atmosphere
https://youtu.be/KNUvM8wF-Mg
There are five important processes that make up the water cycle.
1. Condensation - the opposite
of evaporation. Condensation occurs when a gas is changed into a liquid.
2. Accumulation/Runoff - Much of the
water that returns to Earth as precipitation runs off the surface of the land,
and flows downhill into streams, rivers, ponds and lakes.
3. Evaporation - the process
where a liquid, in this case water, changes from its liquid state to a gaseous
state.
4. Precipitation - When the
temperature and atmospheric pressure are right, the small droplets of water in
clouds form larger droplets and precipitation occurs. The raindrops fall to
Earth.
5. Transpiration - As plants
absorb water from the soil, the water moves from the roots through the stems to
the leaves. Once the water reaches the leaves, some of it evaporates from the
leaves, adding to the amount of water vapor in the air. This process of
evaporation through plant leaves is called transpiration.
 |
| The Water Cycle |
Bill Nye: Water Cycle
Storms
1. What 3 things
cause storms?
Heat
of the sun, water in the air and spin of the Earth.
- Storms are ___Extreme weather
- What occurs
over the Pacific that effects worldwide weather?
El
Nino: a mysteriously warm body of water
- Which band of
wind stops?
Trade
winds
- Where do
hurricanes get their spin?
The
spin of the Earth
- Electricity is
the __Flow of electrons
- What should you
do if you are in a field during a thunderstorm?
Get
short and make as little contact with the ground.
- How much hotter
is lightning than the sun?
Five
times hotter
- What is the
great spot on Jupiter?
A
hurricane.
- Why doesn’t it
die down?
Jupiter
is huge and spinning fast.
Bill Nye Storms on YouTube
Weather Definitions
Ø Air Pressure: The force with which air pushes against
Earth’s surface.
Ø Atmosphere: The air that surrounds Earth.
Ø Barometer: An instrument for measuring air pressure.
Ø Climate: The long term, average or usual weather
conditions for an area.
Ø Condense: To change from a vapour or gas to a liquid.
Ø Evaporate: To change into a gas or vapour.
Ø Front: the forward edge of a moving mass of warm or
cool air; the place where two air masses meet.
Ø Greenhouse gases: Gases that absorb heat in the atmosphere.
Ø Mesosphere: The layer of the Earth’s atmosphere just
above the stratosphere, extending to about 80 km above the surface of Earth.
Ø Ozone: An unstable form of oxygen that is created
when oxygen comes in contact with UV rays; ozone forms a protective layer in
the stratosphere.
Ø Pollutant: a substance, often a waste material such as
smoke or dust that spoils an environment.
Ø Stratosphere: a layer of the atmosphere, beginning about 16
km up, in which temperatures are more or less uniform and clouds are rare.
Ø Thermosphere: a layer of the atmosphere above the
mesosphere, extending between about 200 km and about 500 km above the surface
of Earth.
Ø Troposphere: The layer of the atmosphere extending from
Earth’s surface to the stratosphere.
This is where weather happens.
Ø UV rays: Invisible, high-energy rays from the sun that
can damage unprotected eyes and skin; also called ultraviolet rays.
Ø Water vapour: Water in its gaseous form.
Ø Weather: The air conditions outside a certain time and
place.
Ø Wind-chill factor: A temperature that is a measure of how cold
the air feels to human skin, taking into account the chilling effect of the
wind, as well as the actual air temperature.